Innovation Outlook: Renewable Ammonia
Ammonia is one of the seven basic chemicals used to produce all other chemical products. It is the second most produced chemical by mass, after sulphuric acid. Around four-fifths of all ammonia is used to produce nitrogen fertilisers, such as urea and ammonium nitrate; as such, it supports food production for around half of the global population.
While renewable ammonia’s use as a carbon-free fuel and hydrogen carrier has been proposed, this has not yet been implemented at significant scale. Renewable ammonia has been produced at an industrial scale using hydropower since 1920; however, most ammonia today is produced from natural gas (72%) and coal (22%).
Jointly developed by the International Renewable Energy Agency (IRENA) and the Ammonia Energy Association (AEA), this report provides a detailed overview of renewable ammonia in contrast to conventional and fossil-based ammonia with carbon mitigation, and includes a review of the current technology status and outlook.
The ammonia production industry produces annual emissions of 0.5 gigatonnes (Gt) of carbon dioxide (CO2), representing around 1% of global CO2 emissions and 15-20% of the chemical sector’s CO2 emissions. Addressing emissions from ammonia production is therefore a key component of the decarbonisation of the chemical and agricultural sectors.
In addition to fostering the development of new renewable ammonia plants, the gradual and increasing co-production of renewable ammonia in existing fossil-based ammonia plants should be stimulated to begin decarbonising current ammonia production assets at an early stage.
Significant new markets are expected to develop over the coming decades for ammonia as a hydrogen carrier, as a fuel for stationary power and heat, and as a transport fuel, particularly in the maritime industry. For these new markets to materialise, large additional volumes of ammonia will be required – with demand in 2050 projected to be roughly three times that of 2020 – and these volumes must be low-carbon.

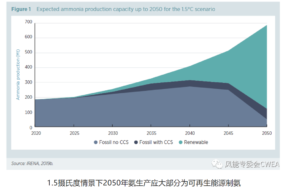
Source: IRENA